OES and GC/MS Study of RF Plasma of Xylenes

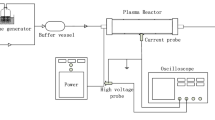
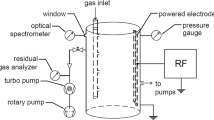
The radio-frequency discharge of xylene isomers was monitored with optical emission spectroscopy (OES). It was found that the meta isomer showed relatively stronger excimer to monomer intensity ratio than the other two isomers. OES also indicates the formation of xylyl (methylbenzyl) radicals. The reaction products of low pressure xylene plasmas were analyzed by gas-chromatography mass spectrometry (GC/MS). It showed that the main composition of the reaction products was 1,2-di-p-tolylethane (DPTE), regardless the types of xylene isomers used. It is known that o- and m-xylyl radicals can undergo rearrangement and convert to p-xylyl radicals. Similar to the cases in benzene and toluene plasmas, the recombination reaction between two p-xylyl radicals is believed to be responsible for the formation of DPTE. Density functional theory calculations suggest that the direct conversion of xylene excimers to DPTE is unlikely.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save
Springer+ Basic
€32.70 /Month
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Buy Now
Price includes VAT (France)
Instant access to the full article PDF.
Rent this article via DeepDyve
Mechanisms of Xylene Isomer Oxidation by Non-thermal Plasma via Paired Experiments and Simulations
Article 09 April 2019
Characterization of Gaseous Plasma Sustained in Mixtures of HMDSO and O2 in an Industrial-Scale Reactor
Article 04 September 2019
Quantum Chemical Approach for Determining Degradation Pathways of Phenol by Electrical Discharge Plasmas
Article 19 November 2016
References
- Nunez CM, Ramsey GH, Ponder WH, Abbott JH, Hamel LE, Kariher PH (1993) Control Technol 43:242 CASGoogle Scholar
- Yamamoto T, Ramanathan K, Lawless PA, Ensor DS, Newsome JR (1992) IEEE Trans Ind Appl 28:528 ArticleCASGoogle Scholar
- Koutsospyros AD, Yin SM, Christodoulatos C, Becker K (2005) IEEE Trans Plasma Sci 33:42 ArticleCASGoogle Scholar
- Schmid S, Jecklin MC, Zenobi R (2010) Chemosphere 79:124 ArticleCASGoogle Scholar
- Saulich K, Müller S (2013) J Phys D Appl Phys 46:045201 ArticleGoogle Scholar
- Zhu XB, Gao X, Yu XN, Zheng CH, Tu X (2016) Catal Today 256:108 ArticleGoogle Scholar
- Föglein KA, Babievskaya I, Szabó PT, Szépvölygi J (2003) Plasma Chem Plasma Process 23:233 ArticleGoogle Scholar
- Christophorou LG, Abu-Zeid MEM, Carter JG (1968) J Chem Phys 49:3775 ArticleCASGoogle Scholar
- Suhr H (1972) Angew Chem Int Ed 11:781 ArticleCASGoogle Scholar
- Oda T, Takahashi T, Yamaji K (2002) IEEE Trans Ind Appl 38:873 ArticleCASGoogle Scholar
- Urashima K, Chang JS (2000) IEEE Trans Dielect Elect Insul 7:602 ArticleCASGoogle Scholar
- Lee S, Chen HF, Chin CJ (2007) J Appl Phys 101:113303 ArticleGoogle Scholar
- Lee S, Chen HF, Peng JW (2007) Plasma Chem Plasma Process 27:256 ArticleCASGoogle Scholar
- Lee S, Liu SJ, Liang RJ (2008) J Phys Chem A 112:13500 ArticleCASGoogle Scholar
- Amicangelo JC (2005) J Phys Chem A 109:9174 ArticleCASGoogle Scholar
- Carter JG, Christophorou LG, Abu-Zeid MEM (1967) J Chem Phys 47:3879 ArticleCASGoogle Scholar
- Greenleaf JR, Lumb MD, Birks JB (1968) J Phys B 1:1157 ArticleGoogle Scholar
- Hirayama F, Lipsky S (1969) J Chem Phys 51:1939 ArticleCASGoogle Scholar
- Birks JB, Braga CL, Lumb MD (1965) Proc Roy Soc A283:83 ArticleGoogle Scholar
- Law KS, Schauer M, Bernstein ER (1984) J Chem Phys 81:4871 ArticleCASGoogle Scholar
- Saigusa H, Morohoshi M, Tsuchiya S (2001) J Phys Chem A 105:7334 ArticleCASGoogle Scholar
- Lortie Y (1957) J Phys Radium 18:520 ArticleCASGoogle Scholar
- Schuler H, Stockburger MZ (1959) Naturforsch 14a:229 CASGoogle Scholar
- Bindley TF, Walker S (1962) Trans Faraday Soc 58:217 ArticleCASGoogle Scholar
- Watts AT, Walker S (1962) J Chem Soc 58:4323 ArticleGoogle Scholar
- Bieg KW (1981) Thin Solid Films 84:411 ArticleCASGoogle Scholar
- Selco JI, Carrick PG (1989) J Mol Spectrosc 137:13 ArticleCASGoogle Scholar
- Frisch MJ et al (2010) Gaussian 09, revision B.01. Gaussian, Inc., Wallingford CT Google Scholar
- Matsugi A, Miyoshi A (2012) Chem Phys Lett 521:26 ArticleCASGoogle Scholar
- Selco JI, Carrick PG (1989) J Mol Spectrosc 173:277 ArticleGoogle Scholar
- Lee S, Chung YJ (2005) J Appl Phys 98:103304 ArticleGoogle Scholar
- Berlman IB (1965) Handbook of fluorescence spectra of aromatic molecules. Academic Press, New York Google Scholar
- Wilson RD, Barton DG, Baertsch CD, Iglesia E (2000) J Catal 194:175 ArticleCASGoogle Scholar
- Akpolat O, Gündüz G (2005) J Appl Sci 5:236 ArticleGoogle Scholar
- Lanewala MA, Bolton AP (1969) J Org Chem 34:3107 ArticleCASGoogle Scholar
- Venugopalan M, Scott TW (1977) Bull Chem Soc Jpn 50:555 ArticleCASGoogle Scholar
- Hemberger P, Trevitt AJ, Gerber T, Ross E, da Silva G (2014) J Phys Chem A 118:3593 ArticleCASGoogle Scholar
- da Silva G, Moore EE, Bozzelli JW (2009) J Phys Chem A 113:10264 ArticleGoogle Scholar
- Hemberger P, Trevitt AJ, Ross E, da Silva G (2013) J Phys Chem Lett 4:2546 ArticleCASGoogle Scholar
- Errede LA, Cassidy JP (1962) J Phys Chem 67:73 ArticleGoogle Scholar
- TOXNET Databases. https://toxnet.nlm.nih.gov/
- Chevron Philips Chemical Company. Material safety data sheet. August 24 2011
Acknowledgments
This work was supported by the Ministry of Science and Technology of Taiwan (MOST 105-2119-M-033-004 and MOST 103-2632-M-033-001-MY3).
Author information
Authors and Affiliations
- Department of Chemistry, Chung Yuan Christian University, Jhongli District, Taoyuan City, 32023, Taiwan Szetsen Lee & Shiao-Jun Liu
- Szetsen Lee



